The iso-corrosion chart below shows the relative
corrosion resistance of several specialty alloys and exotic metals in various concentrations and
temperature of hydrochloric acid (hydrogen chloride). Such alloys will typically be applied when stainless steel shows insufficent corrosion resistance in hydrochloric acid. It can be seen that the corrosion attack of hydrochloric acid, as with most acids, is highly dependent on the temperature.
Chloride containing acids will in many situations show a corrosive nature similar to
hydrochloric acid at comparable acid and/or chloride concentrations.
For precision parts such as
valves, fittings and instrumentation where
tolerances are critical to their operation, a corrosion rate 5 mil/year or larger would typically be considered unacceptable in hydrochoric acid applications.
Hydrochloric acid iso corrosion chart
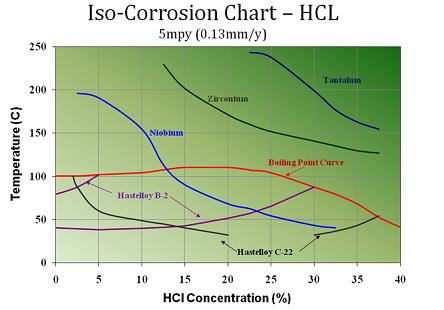
Hydrochloric Acid Iso Corrosion Curves for
Hastelloy (
nickel alloy),
Titanium, Zirconium, Noibium and Tantalum.
Compared with other specialty metals and alloys like
Hastelloy, niobium and
zirconium, the
corrosion resistance of tantalum metal is second to none in hydrochloric acid.
Tantalum metal is an element (atomic number 73) and is considered to be the most corrosion resistant metals commercially available. At temperature less than 150C and a concentration less than 30%, tantalum is considered to have a nil corrosion rate or less than 1 mil/year in chlroride acid service.
The table below shows the relative corrosion resistance in hydrochloric acid at concentrations in the range 5 to 35% and temperatures up to 200C.
