Physical Properties of Zirconium
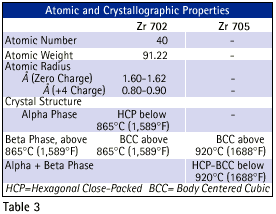
Zirconium alloys are composed of 95.5%to 99.2% zirconium and hafnium with a maximum hafnium content of 4.5%. Zirconium mill products are available in two chemical grades each sharing excellent corrosion resistance and also having slightly different physical properties and mechanical properties. Zirconium 702 is commercially pure zirconium.Zirconium 705 is zirconium alloyed with niobium to increase its strength and improve its formability. Table 1 shows the chemical composition of Zirconium alloys. The presence of hafnium in Zirconium alloys does not significantly influence the physical properties ,mechanical properties, or corrosion properties.
Zirconium, a reactive metal,has a high affinity for oxygen that results in the formation of a protective oxide layer in air at room temperature. This protective oxide gives Zirconium alloys their superior corrosion resistance. The oxide layer can be enhanced through a heat treating process to attain a surface micro-hardness of approximately 480 on the Vickers scale (47 HRC). A properly formed enhanced oxide layer serves as an excellent bearing surface against a variety of materials, imparts impressive erosion resistance in high velocity systems, and can improve the corrosion resistance in certain aggressive environments.
Zirconium alloys exhibit good ductility even at cryogenic temperatures and good strength comparable with other common engineering alloys. In addition to being integral to the oxide layer, oxygen is an interstitial strengthening element in Zirconium alloys. Zirconium alloys do not exhibit a low temperature ductile to brittle transition.
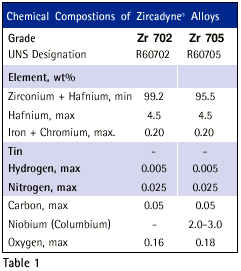
Table 2 lists the thermal properties for Zirconium alloys. Zirconium alloys have a thermal conductivity that is more than 30% higher than those of stainless steel alloys making Zirconium alloys ideal for heat exchangers applications. The linear coefficient of thermal expansion of Zirconium alloys is nearly one-third of the value for stainless steel giving Zirconium alloys superior dimensional stability at elevated temperatures.
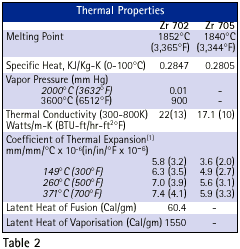
Zirconium 702 has a hexagonal close-packed crystal structure (alpha) below approximately 895 °C (1590 °F) which transforms to a body-centered cubic crystal structure (beta) above this temperature. Zirconium 705 is a two phase system composed of a hexagonal close-packed crystal structure (alpha) and body-centered cubic crystal structure (beta) below approximately 920 °C (1688 °F). Above this temperature, Zirconium 705 transforms to a body-centered cubic crystal structure (beta). Due to the nature of hexagonal close-packed deformation systems (one predominant slip system and three predominant twin systems at typical fabrication temperatures), wrought Zirconium alloys are anisotropic. Table 3 lists the crystallographic characteristics of Zirconium alloys.
Typical wrought and annealed Zirconium alloys exhibit a uniform equiaxed gain structure. All Zirconium alloys mill products are supplied in the annealed condition unless specified otherwise.
Related References:
Zirconium Zirkonium Zircone
Corrosion Resistance of Zirconium
Physical Properties of Zirconium
Zirconium 702 705 Mechanical Properties
Zirconium 702 705 ASME Allowable Stress
Related References:
Physical Properties Constants Table
Physical Properties Table of Metals
Physical Properties of Stainless Steel
Physical Properties of Stainless Steel and Carbon Steel
Physical Properties of Gases at Standard Temperature Pressure
Physical Properties of HDG Hot-Dip Galvanized
Physical Properties of Zirconium
EN 10088-1 Elevated Temperature Physical Properties of Steel
|
