Heat Conduction
Conduction is heat transfer by means of molecular agitation within a material without any motion of the material as a whole. If one end of a metal rod is at a higher temperature, then energy will be transferred down the rod toward the colder end because the higher speed particles will collide with the slower ones with a net transfer of energy to the slower ones. For heat transfer between two plane surface, such as heat loss through the wall of a house, the rate of conduction heat transfer is:
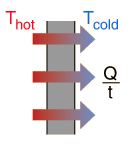 |
| Q |
= heat transferred in time = |
t |
| K |
= thermal conductivity of the barrier |
|
Table Showing Various values for k at 20oC, k = Thermal conductivity (W/mK)
| Metal |
k=Wm-1K-1 |
| Aluminium |
237 |
| Antimony |
18.5 |
| Beryllium |
218 |
| Brass |
110 |
| Cadmium |
92 |
| Cobalt |
69 |
| Constantan |
22 |
| Copper |
398 |
| Gold |
315 |
| Iridium |
147 |
| Cast Iron |
55 |
| Pure Iron |
80.3 |
| Wr't Iron |
59 |
| Lead |
35.2 |
| Magnesium |
156 |
| Molybdenum |
138 |
| Monel |
26 |
| Nickel |
90.5 |
| Platinum |
73 |
| Silver |
427 |
| Carbon Steel |
50 |
| Stainless Steel |
25 |
| Tin |
67 |
| Zinc |
113 |
| Plastics |
|
| Acrylic |
0.2 |
| Nylon 6 |
0.25; |
| Polythene High Den |
0.5 |
| PTFE |
0.25 |
| PVC |
0.19 |
|
