The Importance of the Surface
The seawater corrosion resistance offered by copper-nickel alloys results from the formation of a thin, adherent, protective surface film which forms naturally and quickly upon exposure to clean seawater. The film is complex and predominantly comprises of cuprous oxide, often containing nickel and iron oxide, cuprous hydroxychloride and cupric oxide. The film can be brown, greenish brown or brownish black.
Initial exposure to clean seawater is crucial to the long-term performance of copper-nickel. The initial film forms fairly quickly over the first couple of days but takes 2-3 months to fully mature. Figure 1 shows the rate of film formation on 90-10 copper nickel in seawater at 16°C measured by copper in the effluent of a condenser over a 3-month period after start up. Copper content was found to decrease to one tenth in ten minutes and one hundredth in an hour. After three months, the copper in the effluent was virtually the same level as that of the intake water. Indirectly, this shows that the maturity of the protective film reduced the corrosion rate of the condenser surface.
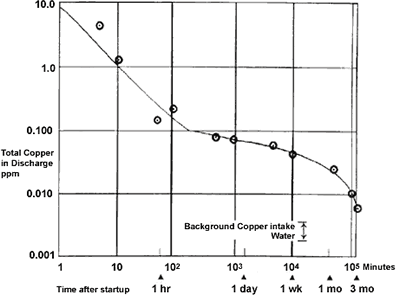 FIGURE 1. Formation rate of corrosion product film on Alloy C70600 in seawater.
FIGURE 1. Formation rate of corrosion product film on Alloy C70600 in seawater.
At higher temperature, the film forms and matures faster. At 27°C, a common inlet temperature for Middle East desalination plants, rapid film formation and good protection can be expected in a few hours. At lower temperatures the process is slower but the film does form even in Arctic and Antarctic waters.
Copper Nickel for Seawater Corrosion Resistance and Antifoulin
90-10 and 70-30 Copper-Nickel Alloys
Corrosion Resistance
The Importance of the Surface
General Corrosion Rates
Localised Corrosion
Velocity Effects
Sand Erosion
Galvanic Properties
Handling Sulfides
Ferrous sulfate treatment
Biofouling Resistance
Ease of Biofouling Removal
Reasons for Biofouling Resistance
Boat Hull Experience
Offshore Sheathing
Conclusions
|
