Handling Sulfides
If exposed to polluted water, especially if this is the first service water to come in contact with the alloy surface, any sulfides present can interfere with surface film formation, producing a black film containing cuprous oxide and sulfide. This is not as protective as films formed in clean water and higher general corrosion rates and pitting can be experienced. The sulfide film can gradually be replaced by an oxide film during subsequent exposure to aerated conditions, although high corrosion rates can be expected in the interim. However, if an established cuprous oxide film is already present, then periodic exposure to polluted water can be tolerated without damage to the film.
Figure 3 examines the behaviour of 90-10 copper nickel in aerated and sulfide polluted waters. In the complete absence of oxygen, corrosion rates are low as indicated by point 1 and current i1 and remained low up to sulfide concentrations as high as 55g/m and velocities of 5m/s. In aerated waters, corrosion rates are somewhat higher, referring to the line AB and current i2. The higher corrosion rate in aerated waters is due to the change from hydrogen reduction to oxygen reduction as the primary cathodic reaction. In polluted waters where both oxygen and sulfide may be present under transient conditions, the cathodic reaction is still oxygen reduction with a much higher corrosion rate, referring to point 2 and current i4. This work illustrates the high corrosion rates are likely to occur in partially deaerated waters with sulfides present and in estuarine waters where there is alternate exposure to aerated waters and partially de-aerated waters with each tide change.
For condenser systems, it is the fitting out and commissioning period when problems are most likely to occur from sulfides . The ideal situation whether in a ship or power plant is to recirculate aerated, clean seawater at initial start up for sufficient time to form a good protective film(4,19,20). When formed, this provides a high degree of corrosion protection to subsequent sulfides. In situations where it is not possible to use clean seawater, circulating the system initially with fresh water containing ferrous sulfate additive will encourage effective film formation.
When outfitting in sulfide polluted waters, piping and condensers should be hydrotested with local fresh waters if possible. If hydrotesting must be done in polluted waters, it should be scheduled as late in construction and outfitting as possible. The hydrotest water should be drained after hydrotesting if possible to minimize sulfide attack or the use of ferrous sulfate or other inhibitors can be considered if draining is impractical. The system should be placed in service in clean water as soon as possible.
If polluted seawater is introduced at start up in condensers, it is important to keep it circulating. The system should be drained and air blown dry for standby periods of 3-4 days or more. A similar situation exists at shutdowns.
For other situations where there is brief exposure to sulfides during normal operating service, clean water should be returned to as soon as possible. Normal harbour turn around times which often involve exposure to polluted water have rarely led to significant problems. However, it is beneficial for a flow through the condenser to be maintained, even if necessary at a reduced level, when in port.
Sulfides are present in polluted water either as industrial effluent or when the water conditions support the growth of sulfate reducing bacteria. They can also occur in stagnant conditions as a result of decomposition of organic matter. Exposure to sulfides should be restricted wherever possible and particularly during the first few months of contact with seawater while the oxide film is maturing.
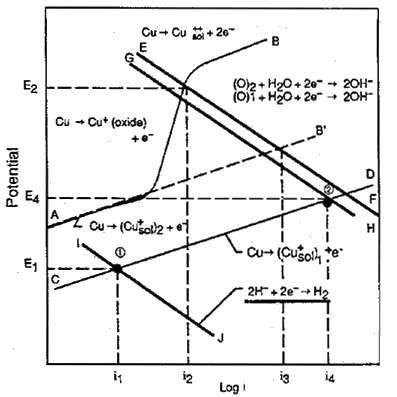 FIGURE 3. Influence of sulfide and oxygen on the corrosion current in a copper-nickel alloy exposed to flowing seawater.
FIGURE 3. Influence of sulfide and oxygen on the corrosion current in a copper-nickel alloy exposed to flowing seawater.
In situations where the metal surface becomes exposed to sulfides under deposits or sediment caused by sulfate reducing bacteria e.g. where deposits are not removed from tubing, the remedy is proper scheduled cleaning. Such cleaning is often scheduled at 2-6 month intervals and accomplished by water flushing or cleaning with non-metallic brushes. Alternatively, sponge ball cleaning is also employed. Such procedures are also necessary to restore optimum heat transfer. Where there is long term exposure to deaerated sulfide containing seawater or regular alternating exposure to sulfide polluted water and aerated waters, copper-nickel is generally not recommended.
Copper Nickel for Seawater Corrosion Resistance and Antifoulin
90-10 and 70-30 Copper-Nickel Alloys
Corrosion Resistance
The Importance of the Surface
General Corrosion Rates
Localised Corrosion
Velocity Effects
Sand Erosion
Galvanic Properties
Handling Sulfides
Ferrous sulfate treatment
Biofouling Resistance
Ease of Biofouling Removal
Reasons for Biofouling Resistance
Boat Hull Experience
Offshore Sheathing
Conclusions
|
