HDG Hot Dip Galvanized Coating Thickness
The American Society of Testing and Materials International (ASTM), the Canadian Specification Association (CSA) and the American Association of State Highway and Transportation Officials (AASHTO) specifications establish minimum standard for thickness of galvanized coatings on various categories of items. These minimum standard are routinely exceeded by galvanizers due to the nature of the galvanizing process.
Factors influencing the thickness and appearance of the galvanized coating include chemical composition of the steel, steel surface condition, cold-working of steel prior to galvanizing, bath temperature, bath immersion time, bath withdrawal rate, and steel cooling rate.
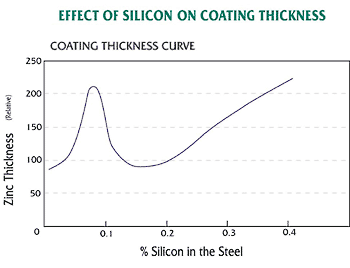
The chemical composition of the steel being galvanized is very important. The amount of silicon and phosphorus in the steel strongly influences the thickness and appearance of the galvanized coating. Silicon, phosphorous or combinations of the two elements can cause thick, brittle galvanized coatings. The coating thickness curve shown in the figure below relates the effect of silicon in the base steel to the thickness of the zinc coating. The carbon, sulfur and manganese content of the steel also may have a minor effect on the galvanized coating thickness.
The combination of elements mentioned above, known as “reactive steel” in the galvanizing industry, tends to accelerate the growth of zinc-iron alloy layers. This may result in a finished galvanized coating consisting entirely of zinc-iron alloy. Instead of a shiny appearance, the galvanized coating will have a dark gray, matte finish. This dark gray, matte coating will provide as much corrosion protection as a galvanized coating having a bright appearance.
Even though it is not a part of the controlled composition of the steel, silicon may be present in many steels commonly galvanized. This occurs primarily because silicon is used in the deoxidization process in steel making and is found in continuously cast steel. The phosphorus content should never be greater than 0.04% for steel that is intended for galvanizing. Phosphorus acts as a catalyst during galvanizing, resulting in rapid growth of the zinc-iron alloy layers. This growth is virtually uncontrollable during the galvanizing process.
As the galvanizing reaction is a diffusion process, higher zinc bath temperatures and longer immersion times generally will produce somewhat heavier alloy layers. Like all diffusion processes, the reaction proceeds rapidly at first and then slows as layers grow and become thicker. However, continued immersion beyond a certain time will have little effect on further coating growth. When galvanizing reactive steels, the diffusion process proceeds at a faster rate, producing thicker coatings.
The thickness of the outer pure zinc layer is largely dependent upon the rate of withdrawal from the zinc bath. A rapid rate of withdrawal causes an article to carry out more zinc and generally results in a thicker coating.
ASTM, CSA and AASHTO specifications and inspection standards for galvanizing recognize that variations occur in both coating thickness and compositions. Thickness specifications are stated in average terms. Further, coating thickness measurements must be taken at several points on each inspected article to comply with ASTM A 123/A 123M for structural steel and A 153/A 153M for hardware.
It is difficult to provide precise guidance in the area of steel selection without qualifying all of the grades of steel commercially available. The guidelines discussed below usually result in the selection of steels that provide good galvanized coatings.
- Levels of carbon less than 0.25%, phosphorus less than 0.04%, or manganese less than 1.35% are beneficial
- Silicon levels less than 0.04% or between 0.15% and 0.25% are desirable

Related References:
1. About Zinc
2. About Hot-Dip Galvanizing
3. HDG Hot-Dip Galvanizing Last Time
4. Cost of Galvanized Steel
5. Selection of Zinc Coatings
6. Zinc Coatings-Galvanized|Electrogalvanized|Galvanneal|Galfan
7. Physical Properties of HDG Hot-Dip Galvanized
8. HDG Hot-Dip Galvanized Abrasion Resistance Resistance to Mechanical Damage
9. Hot-Dip Galvanized Corrosion Protection and the Zinc Patina
10. HDG Hot-Dip Galvanized High Temperature Exposure
11. HDG Hot-Dip Galvanized Surface Reflectivity
12. HDG Hot Dip Galvanized Coating Structure
13. HDG Hot Dip Galvanized Bond Strength
14. HDG Hot Dip Galvanized Coating Uniformity
15. HDG Hot Dip Galvanized Coating Thickness
16. Powder Coating Hot Dipped Galvanized Steel
17. Painting Hot-Dippped Galvanized Steel
18. Painting Hot-Dipped Galvanized Steel Surface Preparation
19. Surface Coatings for Corrosion
20. Hot-Dip Galvanizing Surface Preparation
21. Hot-Dip Galvanizing Galvanizing
22. Hot-Dip Galvanizing Inspection
23. Characteristics of Zinc
24. Hot-Dip Galvanizing Performance in Atmosphere
25. Hot-Dip Galvanizing in Atmosphere Time to First Maintenance
26. Hot-Dip Galvanizing Performance in Soil
27. Soil Corrosion Data for Corrugated Steel Pipe
28. Hot-Dip Galvanizing Performance in Water
29. Cause of Zinc Corrosion
30. Corrosion of Zinc Coated Steel in Selected Natural Fresh Water
31. Corrosion of Zinc and Zinc Coated Steel in Sea Water
32. Corrosion of Zinc Coating in Industrial and Domestic Water
33. Concrete Corrosion of Hot Dip Galvanizing
34. Concrete corrosion resistance of hot dip galvanized reinforcing
35. Removal of Forms Concrete Corrosion
36. Zinc Reaction in Concrete Corrosion
37. Concrete Corrosion References
38. Hot-Dip Galvanizing Performance in Chemical Solutions
39.Hot-Dip Galvanizing Performance in Contact with Other Metals
40. Hot-Dip Galvanizing Performance in contact with Treated Wood
41. Hot-Dip Galvanizing Performance in contact with Food
42. Hot-Dip Galvanizing Performance in Extreme Temperature
|
