Hot-Dipped Galvanized Corrosion Protection and Zinc Patina
Freshly galvanized steel progresses through a natural weathering process. During the first few weeks after an article has been galvanized, it develops a natural protective patina. If allowed to develop properly, the patina itself provides a corrosion protection layer for the active zinc metal.
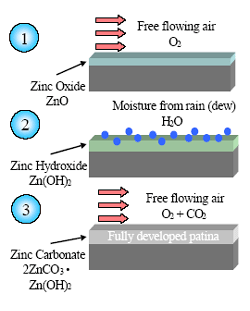
The formation of the zinc patina begins with the development of a thin layer of zinc oxide particulates on the freshly coated surface. These particulates react with water, from rainfall or dew, to form a porous, gelatinous zinc hydroxide. During drying, this product reacts with carbon dioxide present in the atmosphere and converts into a thin, compact and tightly adherent layer of corrosion products consisting mainly of basic zinc carbonate. The rate of patina formation varies according to the environmental conditions. Typically, it takes approximately 6-12 months to fully develop.
Handling and storage conditions can inhibit the formation of the patina. Storage areas that are high in humidity and lack air circulation tend to promote excessive growth of zinc oxide and zinc hydroxide. Adequate ventilation must be provided so that the build-up and retention of excessive water on the surface of the galvanized steel are avoided.
Related References:
1. About Zinc
2. About Hot-Dip Galvanizing
3. HDG Hot-Dip Galvanizing Last Time
4. Cost of Galvanized Steel
5. Selection of Zinc Coatings
6. Zinc Coatings-Galvanized|Electrogalvanized|Galvanneal|Galfan
7. Physical Properties of HDG Hot-Dip Galvanized
8. HDG Hot-Dip Galvanized Abrasion Resistance Resistance to Mechanical Damage
9. Hot-Dip Galvanized Corrosion Protection and the Zinc Patina
10. HDG Hot-Dip Galvanized High Temperature Exposure
11. HDG Hot-Dip Galvanized Surface Reflectivity
12. HDG Hot Dip Galvanized Coating Structure
13. HDG Hot Dip Galvanized Bond Strength
14. HDG Hot Dip Galvanized Coating Uniformity
15. HDG Hot Dip Galvanized Coating Thickness
16. Powder Coating Hot Dipped Galvanized Steel
17. Painting Hot-Dippped Galvanized Steel
18. Painting Hot-Dipped Galvanized Steel Surface Preparation
19. Surface Coatings for Corrosion
20. Hot-Dip Galvanizing Surface Preparation
21. Hot-Dip Galvanizing Galvanizing
22. Hot-Dip Galvanizing Inspection
23. Characteristics of Zinc
24. Hot-Dip Galvanizing Performance in Atmosphere
25. Hot-Dip Galvanizing in Atmosphere Time to First Maintenance
26. Hot-Dip Galvanizing Performance in Soil
27. Soil Corrosion Data for Corrugated Steel Pipe
28. Hot-Dip Galvanizing Performance in Water
29. Cause of Zinc Corrosion
30. Corrosion of Zinc Coated Steel in Selected Natural Fresh Water
31. Corrosion of Zinc and Zinc Coated Steel in Sea Water
32. Corrosion of Zinc Coating in Industrial and Domestic Water
33. Concrete Corrosion of Hot Dip Galvanizing
34. Concrete corrosion resistance of hot dip galvanized reinforcing
35. Removal of Forms Concrete Corrosion
36. Zinc Reaction in Concrete Corrosion
37. Concrete Corrosion References
38. Hot-Dip Galvanizing Performance in Chemical Solutions
39.Hot-Dip Galvanizing Performance in Contact with Other Metals
40. Hot-Dip Galvanizing Performance in contact with Treated Wood
41. Hot-Dip Galvanizing Performance in contact with Food
42. Hot-Dip Galvanizing Performance in Extreme Temperature
|
