Concrete corrosion resistance of hot dip galvanized reinforcing
Under normal conditions, concrete is alkaline (PH of about 12.5) due to the presence of calcium hydroxide. In such an environment, a passivating iron-oxide film forms on the steel, causing almost complete corrosion inhibition. As the pH of the concrete surrounding the reinforcement is reduced by the intrusion of salts, leaching, or carbonation, the system becomes active and corrosion proceeds.
The presence of chloride ions can affect the inhibitive properties of the concrete in two ways. The presence of chloride ions creates changes in the iron oxide, resulting in pitting corrosion. Carbonation can lower the pH and increase the corrosion rate. Any additional lowering of the pH will accelerate the bare steel corrosion rate.
The iron corrosion products that form on steel have much greater volume than the metal that is consumed in the corrosion reaction. This increase in volume around the bare steel rebar exerts great disruptive tensile stress on the surrounding concrete. When resultant tensile strength is greater than the concrete tensile strength, the concrete cracks (as shown in the figure below), leading to more changes by allowing rain and chlorides direct access to the steel bar. Corrosion cracks are usually parallel to the reinforcement and are quite distinct from transverse cracks associated with tension in the reinforcement caused by loading. As the corrosion proceeds, the longitudinal cracks widen and, together with structural transverse cracks, cause spalling of the concrete.
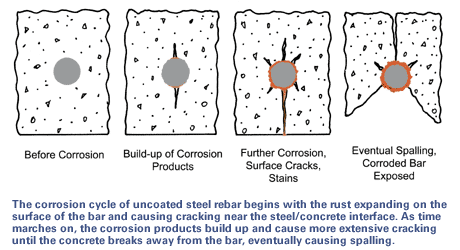
The reason for the extensive use of hot-dip galvanized steel is the twofold nature of the coating. As a barrier coating, galvanizing provides a tough, metallurgically bonded zinc coating that completely covers the steel surface, sealing it from the corrosive action of the environment. Additionally, zinc’s sacrificial (cathodic) action protects the steel even where damage or minor discontinuity occurs in the coating.
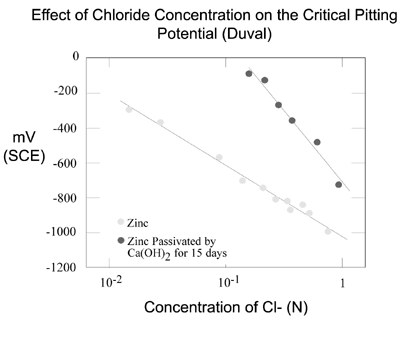
Zinc is characterized by its amphoteric nature and its ability to passivate due to the formation of protective reaction product films. Reaction of zinc with fresh cement leads to passivity by formation of a diffusion barrier layer of zinc corrosion products. Comparison of the two lines in the figure below emphasizes the importance of the passivating layer for corrosion protection against chlorides. The corrosion potential is much lower for zinc that has been passivated, prior to exposure to chlorides, than zinc that has not been passivated.
Both steel and zinc are normally passive in the highly alkaline environment of concrete. However, penetration of chloride ions to the metal surface can break down this passivity and initiate rusting of steel or sacrificial corrosion of the zinc. The susceptibility of concrete structures to the intrusion of chlorides is the primary incentive for using galvanized steel reinforcement.
Galvanized reinforcing steel can withstand exposure to chloride ion concentrations several times higher (at least 4 to 5 times) than the chloride level that causes corrosion in black steel reinforcement. While black steel in concrete typically depassivates below a pH of 11.5, galvanized reinforcement can remain passivated at a lower pH, thereby offering substantial protection against the effects of concrete carbonation.
These two factors combined – chloride tolerance and carbonation resistance – are widely accepted as the basis for superior performance of galvanized reinforcement compared to black steel reinforcement. The total life of a galvanized coating in concrete is made up of the time taken for the zinc to depassivate (which is longer than that for black steel, because of its higher tolerance to chloride ions and carbonation resistance), plus the time taken for the dissolution of the alloy layers in the zinc coating. Only after the coating has fully dissolved in a region of the bar will localized corrosion of the steel begin.
Galvanizing protects the steel during in-plant and on-site storage, as well as after embedment in the concrete. In areas where the reinforcement may be exposed due to thin or porous concrete, cracking, or damage to the concrete, the galvanized coating provides extended protection. Since zinc corrosion products occupy a smaller volume than iron corrosion products, the corrosion that may occur to the galvanized coating causes little or no disruption to the surrounding concrete. Tests also confirm that zinc corrosion products are powdery, non-adherent and capable of migrating from the surface of the galvanized reinforcement into the concrete matrix, reducing the likelihood of zinc corrosion-induced spalling of the concrete (Yeomans).
Related References:
1. About Zinc
2. About Hot-Dip Galvanizing
3. HDG Hot-Dip Galvanizing Last Time
4. Cost of Galvanized Steel
5. Selection of Zinc Coatings
6. Zinc Coatings-Galvanized|Electrogalvanized|Galvanneal|Galfan
7. Physical Properties of HDG Hot-Dip Galvanized
8. HDG Hot-Dip Galvanized Abrasion Resistance Resistance to Mechanical Damage
9. Hot-Dip Galvanized Corrosion Protection and the Zinc Patina
10. HDG Hot-Dip Galvanized High Temperature Exposure
11. HDG Hot-Dip Galvanized Surface Reflectivity
12. HDG Hot Dip Galvanized Coating Structure
13. HDG Hot Dip Galvanized Bond Strength
14. HDG Hot Dip Galvanized Coating Uniformity
15. HDG Hot Dip Galvanized Coating Thickness
16. Powder Coating Hot Dipped Galvanized Steel
17. Painting Hot-Dippped Galvanized Steel
18. Painting Hot-Dipped Galvanized Steel Surface Preparation
19. Surface Coatings for Corrosion
20. Hot-Dip Galvanizing Surface Preparation
21. Hot-Dip Galvanizing Galvanizing
22. Hot-Dip Galvanizing Inspection
23. Characteristics of Zinc
24. Hot-Dip Galvanizing Performance in Atmosphere
25. Hot-Dip Galvanizing in Atmosphere Time to First Maintenance
26. Hot-Dip Galvanizing Performance in Soil
27. Soil Corrosion Data for Corrugated Steel Pipe
28. Hot-Dip Galvanizing Performance in Water
29. Cause of Zinc Corrosion
30. Corrosion of Zinc Coated Steel in Selected Natural Fresh Water
31. Corrosion of Zinc and Zinc Coated Steel in Sea Water
32. Corrosion of Zinc Coating in Industrial and Domestic Water
33. Concrete Corrosion of Hot Dip Galvanizing
34. Concrete corrosion resistance of hot dip galvanized reinforcing
35. Removal of Forms Concrete Corrosion
36. Zinc Reaction in Concrete Corrosion
37. Concrete Corrosion References
38. Hot-Dip Galvanizing Performance in Chemical Solutions
39.Hot-Dip Galvanizing Performance in Contact with Other Metals
40. Hot-Dip Galvanizing Performance in contact with Treated Wood
41. Hot-Dip Galvanizing Performance in contact with Food
42. Hot-Dip Galvanizing Performance in Extreme Temperature
|
