Tantalum is a refractory metal with a melting point of 5425°F (2996°C). It is a tough, ductile metal, which can be formed into almost any shape. It is used in
corrosion resistant applications in environments no other metal can withstand. The major limitation of
tantalum is its reactivity with oxygen and nitrogen in the air at
temperature above 300°C.
Tantalum is the most
corrosion resistant metal in common use today. The presence of a naturally occurring oxide film on the surface of tantalum is the reason for tantalum's extreme corrosion resistant
properties in aggresive media. The corrosion resistance in
hydrochloric acid and resistance in
sulfuric acid are second to none. It is inert to practically all organic and inorganic compounds. Tantalum's corrosion resistance is very similar to glass as both are unsuitable for use in hydrofluoric acid and strong hot alkali applications.
For this reason tantalum is often used with glass lined steel reactors as patches, dip tubes, piping and overhead condensers. Tantalum is inert to
sulphuric acids nd hydrochloric acid in all concentrations below 300°F. The corrosion attack on tantalum up to 400°F is not significant and is in common use up to 500°F.
Tantalum is not corroded by nitric acid in concentrations up to 98% and
temperature up to at least 212°F. Tantalum has proven itself to be totally inert in many corrosion applications. Some
heat exchanger installations have been in continuous use for over 40 years in multi-product research environments without so much as a gasket change due to corrosion.
By the
Surface Alloy Technology it is possible to apply
corrosion resitant surface layers of pure tantalum on top lower performance substartes like e.g. stainless steel.
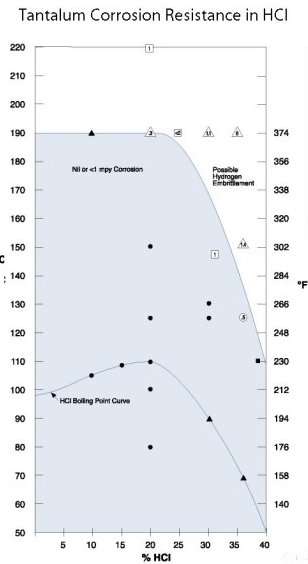
Corrosion of Tantalum in Hydrochloric Acid
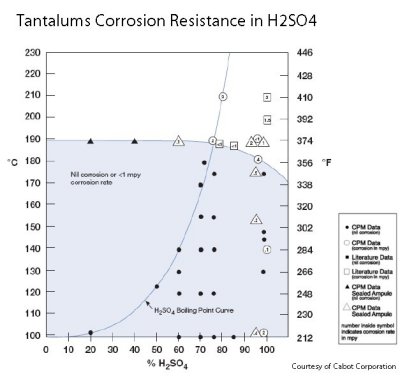
Corroion of Tantalum in Sulfuric Acid
